It’s pitch black, it’s raining and you’re fumbling inside you bag looking for the front door key. It’s got 5 different pockets but no matter how hard you try you just can’t find it. So you’ve got a 1 in 5 chance of finding it … right? You’re getting wetter and wetter. Each pocket has got a ton of other things in them as well but the keys allude you. Are they at the top, bottom or either side of each pocket ? So that now makes 20 possible locations (4 in each pocket). But what about the fact they could hide behind or inside the other objects in the bag. Now the chances of finding them by random fumblings are going up again from 1 in 20 to 1 in 40 ?, 1 in 100 ? The thought crosses your mind “Why do things always seem to go wrong … why me?” You suspect that the universe is out to get you!
In some respects you’re not wrong. But before you get paranoid that there’s some cosmic plot afoot to make your life harder we need to look at some scientific theories surround something called Entropy or the “The laws of thermodynamics”
Entropy & Thermodynamics
Although Thermodynamics is a scientific principle that deals with the fundamental physical quantities of temperature, energy, and entropy that characterize objects inside a closed system it can also be used in a statistical way called probability theory to describe likely outcomes or situations.
In short Entropy is used to quantify the amount of disorder in a system. As disorder increases, entropy increases. If you think of a child’s room after it’s been cleaned then you know that by the end of the week, if they are like most kids, it needs to be cleaned and tidied up again. A teenager’s bedroom is a study in entropy. Statistically speaking there is only a few possible permutations of how the room is arranged that you would consider it to be tidy. However there are far more possible arrangements of the objects in the room that would qualify it as untidy. Given that the closed system (the room) is not static but has energy available to be able to re-arrange itself (in this case a hyperactive kid) then it is statistically more likely to achieve a state of untidiness.
Chaos and Disorder (The 2nd Law of Thermodynamics)
Although there are in effect 4 laws of Thermodynamics the 2nd law serves best here in our somewhat light hearted philosophical debate.
The Second Law of Thermodynamics : The entropy of any closed system not in thermal equilibrium almost always increases. Closed systems spontaneously evolve towards thermal equilibrium — the state of maximum entropy of the system — in a process known as “thermalization”.
Or to paraphrase …
All natural processes tend to go from order (concentrated) to disorder (dispersed).
In other words many processes have a preferred or “natural” direction. Heat flows spontaneously from hot objects to cold ones. Water will flow from high mountains toward rivers. Air rushes out of a punctured rubber balloon. Smells tend to diffuse outward to span greater distances. Humans grow older by the minute. When a house is left unattended, it quickly becomes disorganized. All these processes have one thing in common; they all tend to become more dispersed or chaotic. This a consequence of second law of thermodynamics.
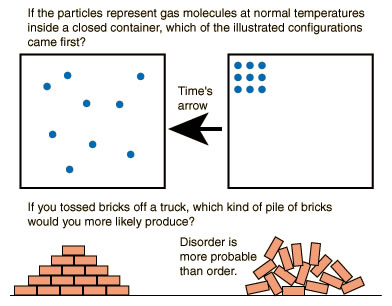 More simply put — events happen in a certain direction. The second law has far-reaching consequences not only in predicting the fate of the universe. In short, it gives us a sense of direction. Thus according to the second law, the universe will continue toward complete disorder (thermodynamic equilibrium), where all non-uniformities in temperature, electric potential, pressure, etcetera, vanish and eventually reach a heat death. Accordingly, it predicts that earthquakes flatten buildings – decreasing order. Similarly, dropping a glass cup off a table edge will likely break it into many pieces, increasing the disorder. Remember that an unbroken glass cup, by the virtue of the careful positioning of the atoms in a lattice structure, is highly ordered.
More simply put — events happen in a certain direction. The second law has far-reaching consequences not only in predicting the fate of the universe. In short, it gives us a sense of direction. Thus according to the second law, the universe will continue toward complete disorder (thermodynamic equilibrium), where all non-uniformities in temperature, electric potential, pressure, etcetera, vanish and eventually reach a heat death. Accordingly, it predicts that earthquakes flatten buildings – decreasing order. Similarly, dropping a glass cup off a table edge will likely break it into many pieces, increasing the disorder. Remember that an unbroken glass cup, by the virtue of the careful positioning of the atoms in a lattice structure, is highly ordered.
In Conclusion …
The arrangement of molecules or objects into what we consider a proper or desirable arrangement or state are statistically small when compared with all the other possible outcomes. So if we go back to the keys in your bag they are unlikely to be in the place you left them given that inside the bag, the closed system, there are many more ways to arrange the items within it than the place you think you left them.
So why am I here when statistically speaking my molecules are more likely to be spread across the universe in a sort of cosmological soup rather than neatly arranged into me ? … a question we all surely ask ourselves at some point in our lives!
For the curious : The 4 laws are ...
There are 4 laws that describe how the quantities of temperature, energy, and entropy behave under various circumstances. They are :
If two systems are in thermal equilibrium with a third system, they must be in thermal equilibrium with each other. This law helps define the notion of temperature.
First law of thermodynamics:Heat and work are forms of energy transfer. While energy is invariably conserved, the internal energy of a closed system changes as heat and work are transferred in or out of it. Equivalently, perpetual motion machines of the first kind are impossible.
Second law of thermodynamics:The entropy of any closed system not in thermal equilibrium almost always increases. Closed systems spontaneously evolve towards thermal equilibrium — the state of maximum entropy of the system — in a process known as “thermalization”. Equivalently, perpetual motion machines of the second kind are impossible.
Third law of thermodynamics:The entropy of a system approaches a constant value as the temperature approaches zero. The entropy of a system at absolute zero is typically zero, and in all cases is determined only by the number of different ground states it has.




You must be logged in to post a comment.