The answer to this one is in fact changing …
If you mean the light bulb that has lit up our homes since the 1800s that is the rather inefficient incandescent bulb then :
In a Nutshell : (Incandescent) Light bulbs light up because electrons have to squeeze through their narrow filament, forcing them to bash into the atoms of the filament, which makes them vibrate and give off heat and light.
OR if you mean the newer more efficient LED Bulbs …
In a Nutshell : (LED) Light bulbs light up because a flow of electrons move in a semiconductor from a negative layer into holes in a positive layer and give off light in the process.
 The Incandescent Bulb
The Incandescent Bulb
An electrical circuit with a battery, wires and a light bulb is like an aquarium with a water pump, tubes and a filter. The battery acts like a pump, forcing water around the tubes and through the filter, which is like a light bulb. The tubes let the water flow quite easily, but the filter is narrow and partially blocked, so it is harder for the water to get through. Squeezing through causes the water to lose energy by friction.
In an electrical circuit the electrons that carry the current are like the water. They can pass through the wires quite easily because they are made of copper and have a low resistance to electricity, but the filament is made of tungsten, a metal with a high resistance. Also the filament is extremely long and thin- in a typical 60-watt bulb the tungsten filament is 2m long but only a quarter of a millimetre thick, which makes it even more resistant. Resistance is a measure of how easily electricity can pass through something. The electrons work so hard to get through the tungsten that they pass energy to the atoms of the filament. As the tungsten atoms build up energy they get excited, and when they get excited they give off heat and light energy.
 The LED Bulb
The LED Bulb
In the last few years LED replacement bulbs have become much more common.
Unlike ordinary incandescent bulbs, they don’t have a filament that will burn out, and they don’t get especially hot. They are illuminated solely by the movement of electrons in a semiconductor material, and they last just as long as a standard transistor. The lifespan of an LED surpasses the short life of an incandescent bulb by thousands of hours.
The Basics
Let’s begin with the basics.
 An LED is what’s called a “solid-state lighting” technology, or SSL. Basically, instead of emitting light from a vacuum (as in an incandescent bulb) an SSL emits light from a piece of solid matter. In the case of a traditional LED, that piece of matter is a semiconductor.
An LED is what’s called a “solid-state lighting” technology, or SSL. Basically, instead of emitting light from a vacuum (as in an incandescent bulb) an SSL emits light from a piece of solid matter. In the case of a traditional LED, that piece of matter is a semiconductor.
A semiconductor is made of a positively charged and a negatively charged component. The positive layer has “holes” — openings for electrons; the negative layer has free electrons floating around in it. When an electric charge strikes the semiconductor, it activates the flow of electrons from the negative to the positive layer. Those excited electrons emit light as they flow into the positively charged holes.
Stated very simply, an LED produces light when electrons move around within its semiconductor structure.
A Deeper Dive
When No Charge is Applied to the Diode
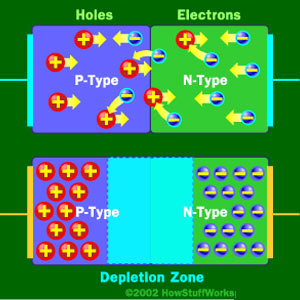
At the junction, free electrons from the N-type material fill holes from the P-type material. This creates an insulating layer in the middle of the diode called the depletion zone.
When Negative End is hooked up to the N-type
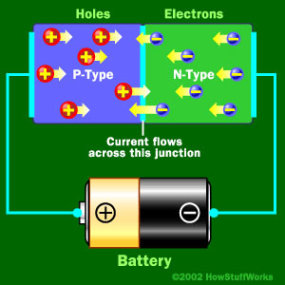
When the negative end of the circuit is hooked up to the N-type layer and the positive end is hooked up to P-type layer, electrons and holes start moving and the depletion zone disappears.
When Negative End is hooked up to the P-type
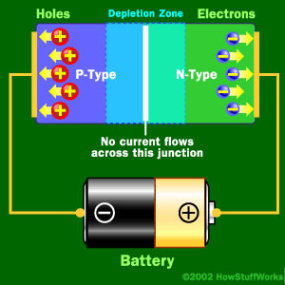
When the positive end of the circuit is hooked up to the N-type layer and the negative end is hooked up to the P-type layer, free electrons collect on one end of the diode and holes collect on the other. The depletion zone gets bigger.
So, broadly speaking, a semiconductor is a material with a varying ability to conduct electrical current. An LED consists of 2 types of semiconductor : an N-Type & a P-Type.
A semiconductor with extra electrons is called N-type material, since it has extra negatively charged particles. In N-type material, free electrons move from a negatively charged area to a positively charged area.
A semiconductor with extra holes is called P-type material, since it effectively has extra positively charged particles. Electrons can jump from hole to hole, moving from a negatively charged area to a positively charged area. As a result, the holes themselves appear to move from a positively charged area to a negatively charged area.
A diode consists of a section of N-type material bonded to a section of P-type material, with electrodes on each end. This arrangement conducts electricity in only one direction. When no voltage is applied to the diode, electrons from the N-type material fill holes from the P-type material along the junction between the layers, forming a depletion zone. In a depletion zone, the semiconductor material is returned to its original insulating state — all of the holes are filled, so there are no free electrons or empty spaces for electrons, and charge can’t flow.
To get rid of the depletion zone, you have to get electrons moving from the N-type area to the P-type area and holes moving in the reverse direction. To do this, you connect the N-type side of the diode to the negative end of a circuit and the P-type side to the positive end. The free electrons in the N-type material are repelled by the negative electrode and drawn to the positive electrode. The holes in the P-type material move the other way. When the voltage difference between the electrodes is high enough, the electrons in the depletion zone are boosted out of their holes and begin moving freely again. The depletion zone disappears, and charge moves across the diode.
If you try to run current the other way, with the P-type side connected to the negative end of the circuit and the N-type side connected to the positive end, current will not flow. The negative electrons in the N-type material are attracted to the positive electrode. The positive holes in the P-type material are attracted to the negative electrode. No current flows across the junction because the holes and the electrons are each moving in the wrong direction. The depletion zone increases.
The interaction between electrons and holes in this setup has an interesting side effect — it generates light!


 Like most metals, tungsten has to be white hot before it gives off much light, so light bulb filaments get to 2,200°C (3, 992°F). Even then, about 90% of their energy is given off as invisible infrared light (heat), so normal light bulbs aren’t very efficient at turning electricity into light.
Like most metals, tungsten has to be white hot before it gives off much light, so light bulb filaments get to 2,200°C (3, 992°F). Even then, about 90% of their energy is given off as invisible infrared light (heat), so normal light bulbs aren’t very efficient at turning electricity into light.
