CAFFEINE HEADS BEWARE
Scientists in Zurich, Switzerland have up with a sensitive new way to analyse the composition of almost any surface, including human skin by blowing a stream of nitrogen across the surface under analysis and collect the gas together with any debris that it dislodges. The material is then fed into a mass spectrometer that can pick apart the chemical composition of anything present on the surface.
Using the technique, say the researchers, they can even detect the difference in caffeine concentrations in an individual before and after a cup of coffee. This means that the approach could be used to monitor drug levels, treatment compliance and address other important questions such as dissecting the chemical make-up of skin samples, plant tissue, frozen meat or other tissues. Critically the process is entirely noninvasive and does not harm the surface under analysis.
So caffeine-heads, watch out, someone could be monitoring your dose quite soon!
How a mass spectrometer works
The basic principle
If something is moving and you subject it to a sideways force, instead of moving in a straight line, it will move in a curve – deflected out of its original path by the sideways force.
The amount of deflection you will get for a given sideways force depends on the mass of the item being struck. If you knew the speed of the item and the size of the force, you could calculate the mass of the item if you knew what sort of curved path it was deflected through. The less the deflection, the heavier the item.
You can apply exactly the same principle to atomic sized particles.
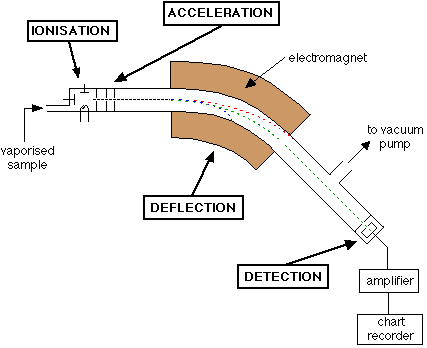
An Outline of What Happens in a Mass Spectrometer
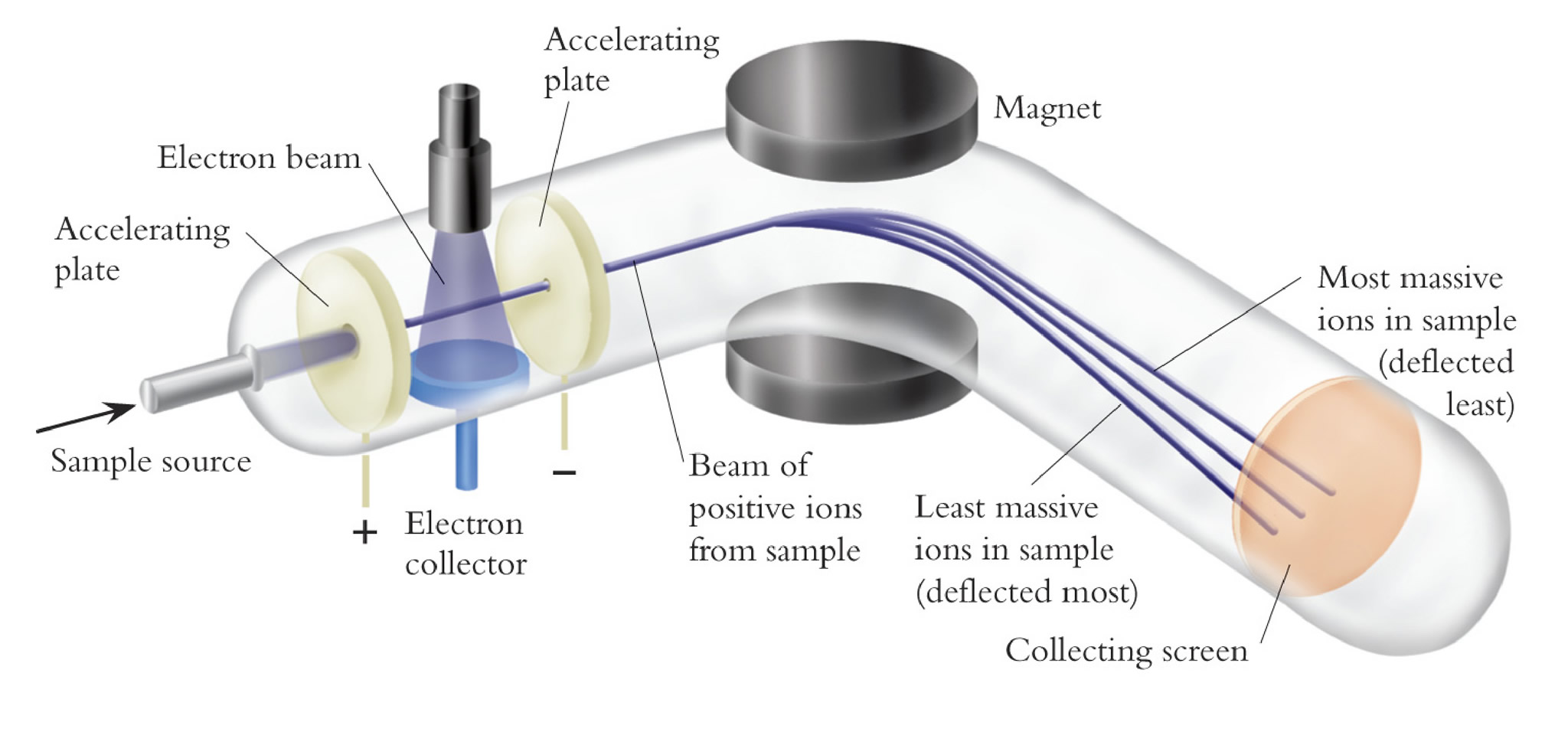
Atoms and molecules can be deflected by magnetic fields – provided the atom or molecule is first turned into an ion. Electrically charged particles are affected by a magnetic field although electrically neutral ones aren’t.
The sequence is :
Stage 1: Ionisation
The atom or molecule is ionised by knocking one or more electrons off to give a positive ion. This is true even for things which you would normally expect to form negative ions (chlorine, for example) or never form ions at all (argon, for example). Most mass spectrometers work with positive ions.
Stage 2: Acceleration
The ions are accelerated so that they all have the same kinetic energy.
Stage 3: Deflection
The ions are then deflected by a magnetic field according to their masses. The lighter they are, the more they are deflected.
The amount of deflection also depends on the number of positive charges on the ion – in other words, on how many electrons were knocked off in the first stage. The more the ion is charged, the more it gets deflected.
Stage 4: Detection
The beam of ions passing through the machine is detected electrically.




You must be logged in to post a comment.